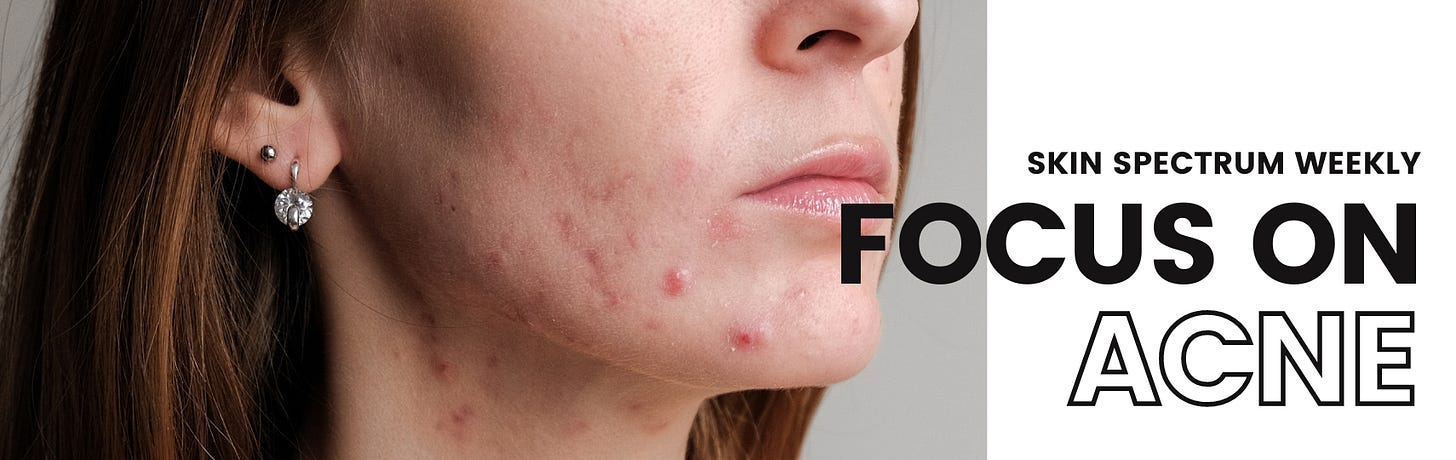
Detachable microneedle delivery of triamcinolone acetonide
Today's report also covers quality of life for family members of acne patients, laser treatment, topical androgen inhibitors, and more (1,300 words, 6 minutes, 50 seconds)
The Focus on Acne e-newsletter is supported by an unrestricted grant from Sun Pharma Canada
Good morning and welcome to the second issue of Focus on Acne presented by Skin Spectrum Weekly. This series provides up-to-date information on developing therapies and trends in acne treatment. We appreciate your feedback and suggestions and invite you to be in touch. Please write to us at health@chronicle.org
Detachable microneedle delivery of triamcinolone acetonide
Findings from a small study suggest that using detachable microneedles (DMNs) to deliver the steroid triamcinolone acetonide is a safe, effective treatment for inflammatory acne while also reducing post-acne erythema.
The findings were published in Clinical, Cosmetic and Investigational Dermatology (June 5, 2023; 16:1431-1441).
The authors of the paper describe DMNs as dissolvable microneedles that detach from a patch base during administration.
In the study, researchers evaluated the acne treatment efficacy and safety of DMNs and DMNs containing triamcinolone acetonide (TA) in 35 patients with facial inflammatory acne. Investigators selected four inflammatory acne lesions from each participant and randomly treated them with one of four modalities: a single application of 700 µm DMNs containing 262.02±15.62 µg TA (700DMNTA), 1,000 µm DMNs containing 160.00±34.92 µg TA (1000DMNTA), 700 µm DMN without TA (700DMN), and a control. They measured efficacy through physical grading, diameter, volume, erythema index, and melanin index. Researchers also assessed reports of adverse effects from patients and physicians.
Investigators observed resolution of inflammatory acne in all three treatment groups significantly earlier than the control group, with median times for resolution of 4.6, 5.25, 6.7, and 8.1 days in the 1000DMNTA, 700DMNTA, 700DMN, and control groups, respectively.
They also found the diameters and post-acne erythema of inflammatory acne were significantly reduced in the treatment groups compared to the control groups. The 1000DMNTA treatment decreased acne size and erythema more than other treatments. DMNTA also tended to decrease acne size and erythema more than DMN with no TA, but there was no statistically significant difference.
All participants preferred DMN over conventional intralesional steroid injection due to less pain and self-application. The researchers observed no adverse effects.
QoL reduced for family members of patients with acne
Patients with acne and their parents have a decreased quality of life (QoL) compared to healthy controls. Assessing QoL in the family in addition to that of the patient may allow improved management of acne vulgaris.
These are the conclusions of a study published in Anales de pediatria (English edition)(June 12, 2023; S2341-2879(23)00130-8).
The authors of the paper note that acne vulgaris is significantly associated with an increased burden of care and has an important impact on the QoL and self-esteem of affected individuals. They conducted this research to assess the QoL of adolescents with acne and their families as well as the association of QoL with acne severity, treatment response, duration of acne and localization of lesions.
Researchers included 100 adolescents with acne vulgaris, 100 healthy controls, and their parents in this study. They gathered data on sociodemographic characteristics, presentation of acne, duration of acne, treatment history, treatment response, and parental sex. Measurement tools used included the Global Acne Severity scale, Children's Dermatology Life Quality Index (CDLQI), and the Family Dermatology Life Quality Index (FDLQI).
They found that in the group of patients with acne, the mean CDLQI score in the patients was 7.89 (SD, 5.43) and the mean FDLQI score in the parents was 6.01 (SD, 6.11). In the control group, the mean CDLQI score in healthy controls was 3.92 (SD, 3.88) and the mean FDLQI score in their family members was 2.12 (SD, 2.91).
The investigators determined there was a statistically significant difference between the acne and control groups in CDLQI and FDLQI scores (p<0.001). There were also statistically significant differences in the CDLQI score based on the duration of acne and the response to treatment.

1,726 nm laser Tx effective for acne across skin types
Findings from a prospective study suggest treatment with a novel 1,726 nm laser is effective and well-tolerated as a treatment for moderate-to-severe acne.
The study was published in the Journal of the American Academy of Dermatology (June 14, 2023; S0190-9622(23)01075-7).
A total of 104 subjects with moderate-to-severe facial acne and Fitzpatrick skin types II to VI were included. Each patient received three laser treatments with the novel device at 3 (-1/+2) week intervals.
The investigators observed a 50% or better reduction in active inflammatory acne lesions in 32.6% of participants four weeks after the final treatment. This increased to 70.8% of patients at 12 weeks and 87.3% at 26 weeks. The percentage of subjects who were clear or almost clear increased from 0% at baseline to 9%, 36.0%, and 41.8% at the 4-, 12- and 26-week follow-up. The researchers observed no serious adverse events related to the device or protocol and the treatments were well tolerated, requiring no anesthetic. Therapeutic outcomes and discomfort were similar across all skin types.
Researchers note that the lack of a control group is a limitation of this study.

Safety and efficacy of topical androgen inhibitor for acne
A new review article, published in the Journal of Drugs in Dermatology (June 1, 2023; 22(6):SF350992s7-SF350992s14) details the mechanism of action, efficacy, and safety of topical clascoterone cream 1%, the first of a new class of topical antiandrogen treatment for acne.
The authors note that clascoterone, also known as cortexolone-17α propionate, is an androgen receptor inhibitor with highly localized activity. Its suspected mechanism of action is competition with dihydrotestosterone (DHT) for androgen receptors located in pilosebaceous units. This in turn inhibits the acnegenic downstream effects of DHT including lipid synthesis and inflammatory cytokine production.
They cite two phase III clinical trials that have shown clascoterone 1% cream applied twice per day to be significantly more effective than placebo cream at treating acne vulgaris in patients ages 12 and older with moderate-to-severe acne.
Clascoterone’s safety profile has also been shown to be similar to that of placebo cream. No systemic antiandrogenic effects were observed in a clinical setting.
The authors conclude that due to its novel mechanism of action and activity limited to the skin, clascoterone presents an opportunity for dermatologists to further optimize care for eligible acne patients, either as a monotherapy or in combination with other anti-acne medications.

Subscribe to The Chronicle of Skin & Allergy
Established in 1995, The Chronicle of Skin & Allergy is a scientific newspaper providing news and information on practical therapeutics and clinical progress in dermatologic medicine.
To apply for a complimentary* subscription, please email health@chronicle.org with your contact information or click the link below.
If you find the contents of this newsletter interesting, please check out the Yadav on Acne podcast. It’s available at Apple iTunes, Stitcher, Spotify, or wherever you get your podcasts.