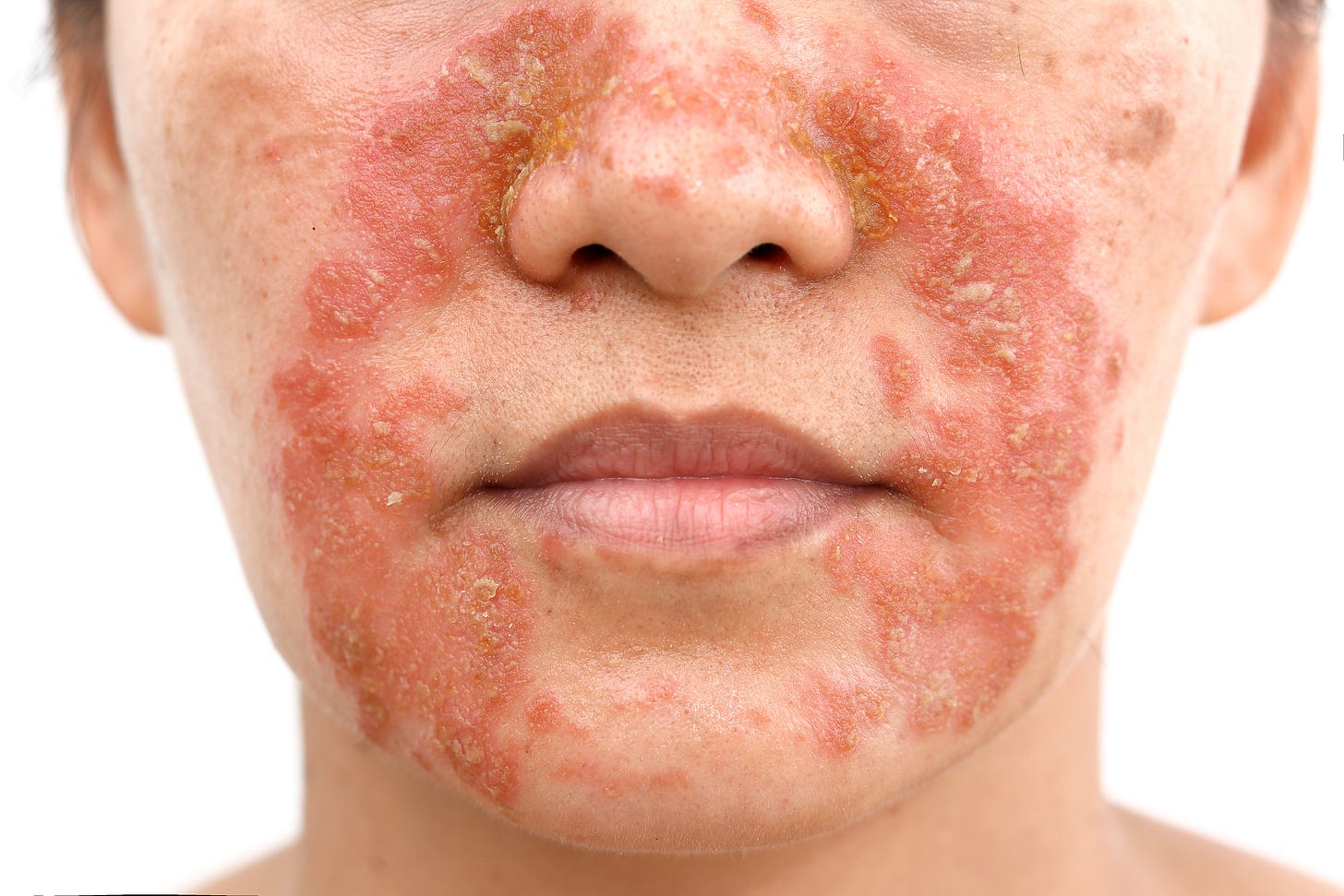
Efficacy and safety of deucravacitinib in Japanese patients with moderate to severe plaque psoriasis
Today's report also covers dactylitis in active psoriatic arthritis patients, risankizumab use, and more (1,900 words, 9 minutes, 30 seconds)
The Vender on Psoriasis e-newsletter is supported by an unrestricted grant from Sun Pharma Canada
Good morning and welcome to the sixth issue of Special Reports: Skin Spectrum Weekly presents “Vender on Psoriasis.” This series is based on Dr. Vender’s popular column appearing in each edition of The Chronicle of Skin & Allergy, which offers expert commentary and opinions on current clinical developments in PsO. We’d love to get your feedback and suggestions and invite you to be in touch. Please write to us at health@chronicle.org

Efficacy and safety of deucravacitinib in Japanese patients with moderate to severe plaque psoriasis
The Journal of Dermatology has published a study examining the effectiveness and safety of deucravacitinib, a selective tyrosine kinase 2 (TYK2) inhibitor, in Japanese patients with moderate to severe plaque psoriasis (Mar. 7, 2023). The study was a subgroup analysis of a global phase 3 trial (POETYK PSO-1; NCT03624127) that included 666 patients. The Japanese patient group consisted of 66 patients who were randomly assigned to treatment with deucravacitinib 6 mg once daily, placebo, or apremilast 30 mg twice daily. Patients who were initially randomized to placebo were crossed over to deucravacitinib at Week 16, while patients randomized to apremilast who did not achieve ≥50% reduction from baseline in Psoriasis Area and Severity Index (PASI 50) score at Week 24 switched to deucravacitinib.
The study found that deucravacitinib demonstrated superior efficacy compared to placebo and apremilast in Japanese patients. The proportion of Japanese patients achieving ≥75% reduction from baseline in PASI (PASI 75) score was numerically higher with deucravacitinib versus placebo and apremilast at Week 16 (78.1% vs. 11.8% and 23.5%, respectively) and versus apremilast at Week 24 (78.1% vs. 29.4%). A numerically higher proportion of patients achieved a static Physician's Global Assessment score of 0 or 1 (clear or almost clear) with at least a two-point improvement from baseline (sPGA 0/1) with deucravacitinib versus placebo or apremilast at Week 16 (75.0% vs. 11.8% and 35.3%) and versus apremilast at Week 24 (75.0% vs. 29.4%). The response rates were maintained through 52 weeks in the deucravacitinib group. The incidence rates for adverse events per 100 person-years (PY) in the Japanese patients were comparable across treatment groups through Week 52 (deucravacitinib, 336.8/100 PY; placebo, 321.0/100 PY; apremilast, 358.6/100 PY), and the most frequently reported adverse event with deucravacitinib was nasopharyngitis. The efficacy and safety of deucravacitinib in Japanese patients were consistent with those in the global population in POETYK PSO-1.
Comment: This new mechanism of action, and once daily oral medication, is certainly an advance over apremilast which has served us quite well for the past few years. However, deucravacitinib showed in head-to-head studies that it was more effective in more patients than apremilast. This study confirms in a Japanese population there is a very good chance of having a patient clear or almost clear from their psoriasis. Adverse events are extremely uncommon and include typical nasopharyngitis. This medication is newly approved in Canada and meets an unmet need for an effective oral medication, especially for those patients who are needle-phobic or would prefer once daily oral therapy. The other advantage of oral therapy is that for patients who travel it is easier to bring through security. Even though several biologics are given every two to three months, for those patients with a very busy lifestyle, a once-a-day pill is extremely convenient.
Guselkumab resolves dactylitis in patients with active psoriatic arthritis
Guselkumab, a selective interleukin-23 p19 subunit inhibitor, has been shown to effectively resolve dactylitis in patients with active psoriatic arthritis, according to pooled results from two phase 3 studies spanning 52 weeks. In previous analyses up to Week 24, significantly higher rates of dactylitis resolution were observed in patients treated with guselkumab compared to placebo. In this study, the researchers investigated the associations between dactylitis resolution and other clinical outcomes over the course of one year. These study results were published in ACR Open Rheumatol (Mar. 7, 2023).
Patients were randomly assigned to receive subcutaneous injections of guselkumab 100 mg at Week 0, Week 4, and then every 4 or 8 weeks, or placebo with crossover to guselkumab at Week 24. Independent assessors determined the severity of dactylitis using a dactylitis severity score (DSS) ranging from 0 to 3 per digit, with a total score ranging from 0 to 60. Dactylitis resolution (DSS = 0) was prespecified, and improvement of at least 20%, 50%, and 70% in DSS from baseline were assessed post hoc through Week 52, with nonresponder imputation for treatment failure through Week 24 and missing data through Week 52. Other clinical outcomes such as ACR50 (American College of Rheumatology 50% improvement), tender/swollen joints, low disease activity (LDA) as assessed by composite indices, and radiographic progression (DISCOVER-2 only) were assessed in patients with and without dactylitis resolution at Week 24 and Week 52.
Results showed that patients with dactylitis at baseline had more severe joint and skin disease compared to those without dactylitis. At Week 52, approximately 75% of patients randomized to receive guselkumab with dactylitis at baseline achieved complete resolution, and approximately 80% showed at least 70% improvement in DSS. New onset dactylitis (DSS ≥1) was uncommon among patients who had a DSS of 0 at baseline through Week 52. Patients who achieved dactylitis resolution with guselkumab were more likely to achieve ACR50, at least 50% reduction in tender and swollen joints, and LDA at both Week 24 and Week 52 compared to those without resolution. At Week 52, patients with dactylitis resolution also showed numerically less radiographic progression from baseline in the DISCOVER-2 study.
Over one year, approximately 75% of patients treated with guselkumab achieved complete resolution of dactylitis associated with improved clinical outcomes. Given the significant burden of dactylitis in patients with psoriatic arthritis, achieving resolution may lead to better long-term patient outcomes.
Comment: It is extremely important that biologics approved for skin psoriasis be also effective with the most common associated disease, psoriatic arthritis. Therefore, it is no surprise that anti IL23 biologics such as guselkumab or risankizumab are both extremely effective for psoriatic arthritis. Certainly, there is controversy when it comes to axial disease because the spine produces its own IL17 and for axial disease, anti-IL17 biologics would be more beneficial. In our dermatology clinic we can often notice dactylitis as we are rarely palpating joints to see if there are tender or swollen areas. Dactylitis is easily recognizable on physical examination. This study shows guselkumab is effective in dactylitis for patients who have psoriatic arthritis, which is extremely important for us to realize as dermatologists. This benefit increases the quality of life of patients by decreasing discomfort associated with these swollen digits. Gone are the days when biologics are chosen only for skin or only for joints. There is improvement in both aspects of psoriatic disease with the biologics that are available today.

Risankizumab Tx for moderate-to-severe psoriasis—A multi-centre, long-term, real-life study from Poland
This multi-centre, long-term, real-life study assessed the efficacy of risankizumab in moderate-to-severe plaque psoriasis. The study, published in the Journal of Clinical Medicine (2023; 12:1675), enrolled 185 patients from 10 dermatologic departments in Poland who received risankizumab treatment. Disease severity was measured using the Psoriasis Area and Severity Index (PASI) at baseline and at defined timepoints of 4, 16, 28, 40, 52, and 96 weeks of treatment. The study evaluated the percentage of patients achieving PASI 90 and PASI 100 responses, as well as the PASI percentage decrease at these timepoints, and analyzed correlations with clinical characteristics and therapeutic effect.
The number of patients evaluated at each defined timepoint was 136, 145, 100, 93, 62, and 22 at 4, 16, 28, 40, 52, and 96 weeks of treatment, respectively. The results showed that at 4, 16, 28, 40, 52, and 96 weeks, PASI 90 response was achieved in 13.2%, 81.4%, 87.0%, 86.0%, 88.7%, and 81.8% of patients, respectively. Similarly, PASI 100 response was achieved in 2.9%, 53.1%, 67.0%, 68.8%, 71.0%, and 68.2% of patients, respectively.
The study revealed a significant negative correlation between the decrease in PASI and the presence of psoriatic arthritis, as well as the patient's age and duration of psoriasis at several timepoints throughout the observation period. These findings suggest that risankizumab may be an effective treatment option for moderate-to-severe plaque psoriasis, with higher PASI 90 and PASI 100 responses observed over time. However, further research is warranted to better understand the long-term efficacy and safety profile of risankizumab.
Comment: The study confirms that a very high PASI 90 is achievable with risankizumab, an extremely effective anti-psoriatic biologic that inhibits IL23. Its efficacy for patients achieving PASI 90 is extremely high and quite often exceeds the average of 70% PASI with ANTI-IL17s. The study confirms that a very high PASI 90 is achievable for patients on risankizumab. In addition to this, in patients that are clear, PASI 100 occurs in over 50% of those treated with risankizumab. My only concern with this study is that its conclusion shows that it “may” be an effective treatment option for patients that have moderate to severe psoriasis. I would suggest that the authors should have made a correction, saying that it “is” an effective treatment for psoriatic patients. That has been proven in many long-term studies as well as real world experience that has been extensively published.
If you find the contents of this newsletter interesting, please check out the Vender on Psoriasis podcast. It’s available at Apple iTunes, Stitcher, Spotify, or wherever you get your podcasts.
Dr. Ron Vender is a Hamilton-based certified dermatologist with 31 years of clinical practice experience and over 100 clinical trials in psoriasis. He is founder and director of Venderm Innovations in Psoriasis, a centre of excellence for Psoriasis offering a comprehensive management solution for individuals with psoriasis.
Subscribe to The Chronicle of Skin & Allergy
Established in 1995, The Chronicle of Skin & Allergy is a scientific newspaper providing news and information on practical therapeutics and clinical progress in dermatologic medicine.
To apply for a complimentary* subscription, please email health@chronicle.org with your contact information or click the link below.






