Efficacy and safety of TYK2/JAK1 inhibitor brepocitinib for active psoriatic arthritis
Today's report also covers clinical improvement speed in real-world settings, biologic therapy exposure during pregnancy, and more (1,700 words, 8 minutes, 20 seconds)
The Vender on Psoriasis e-newsletter is supported by an unrestricted grant from Sun Pharma Canada
Good morning and welcome to the eighth issue of Special Reports: Skin Spectrum Weekly presents “Vender on Psoriasis.” This series is based on Dr. Vender’s popular column appearing in each edition of The Chronicle of Skin & Allergy, which offers expert commentary and opinions on current clinical developments in PsO. We’d love to get your feedback and suggestions and invite you to be in touch. Please write to us at health@chronicle.org

Efficacy and safety of TYK2/JAK1 inhibitor brepocitinib for active psoriatic arthritis
In this study published in Arthritis & Rheumatology, investigators assessed the efficacy and safety of oral brepocitinib in participants with moderately-to-severely active psoriatic arthritis (PsA) for up to 52 weeks. Brepocitinib is a JAKi with selectivity for TYK2 and JAK1 over JAK2 and JAK3 in development for the treatment of several immunological diseases. TYK2 inhibition by brepocitinib provides greater blockade of IL-12 and IL-23 signalling compared with JAK1 inhibition alone; reducing the production of IL-17, one of the major effector cytokines in the pathogenesis of psoriatic disease.
This placebo-controlled, dose-ranging, phase IIb study randomized 218 participants to brepocitinib 10 mg once daily, 30 mg once daily, 60 mg once daily, or placebo, advancing to brepocitinib 30 or 60 mg once daily at week 16. The primary endpoint was American College of Rheumatology (ACR) 20 response rate at week 16. Secondary endpoints included response rates of ACR50/70, 75% and 90% improvement in Psoriasis Area and Severity Index (PASI75/90), and Minimal Disease Activity (MDA) at weeks 16 and 52.
According to the study results, at week 16 the brepocitinib 30 and 60 mg once daily had significantly greater ACR20 response rates (66.7% [p=0.0197] and 74.6% [p=0.0006], respectively), versus placebo (43.3%) and significantly higher ACR50/70, PASI75/90, and MDA response rates. Response rates were maintained or improved through week 52. AEs were mostly mild/moderate; serious AEs (15) in 12 (5.5%) participants included infections in six patients (2.8%) in the brepocitinib 30 and 60 mg once daily groups. No major adverse cardiovascular events or deaths occurred.
Comment: This new class of medication is exciting for the field of dermatology. Gone are the immunosuppressives of the past, and the new focused JAK inhibitors as well as TYK2 inhibitors are being developed. Unfortunately, the JAK inhibition can lead to potential side effects while balancing the efficacy, however, the risk-reward ratio seems to favour these new medications. Quite often these new JAK inhibitors are used for rheumatological disease first and then their indication is transferred over to dermatology. This new molecule showed that there was efficacy in the joints by measuring the ACR and minimal disease activity. However, it was also shown to improve skin responses by measuring PASI 75 and PASI 90. It seems in this small study that adverse events are minimal; however, Phase II studies do not cover enough of a population to determine whether this medication can come to market. Larger Phase III studies are required.
Speed of clinical improvement in the real-world setting from patient-reported Psoriasis Symptoms and Signs Diary: Secondary outcomes from the Psoriasis Study of Health Outcomes through 12 weeks
This study set out to compare the speed of clinical improvement of approved biologics on the symptoms and signs of moderate to severe psoriasis assessed by patients using the validated Psoriasis Symptoms and Signs Diary (PSSD) through 12 weeks (J Eur Acad Dermatol Venereol 2023 May 5. doi: 10.1111/jdv.19161. Online ahead of print).
The researchers assessed the outcomes based on patient reports in the validated Psoriasis Symptoms and Signs Diary (PSSD) through 12 weeks.
Utilizing the PSSD seven-day recall period, patients assessed the symptoms (itch, skin tightness, burning, stinging, and pain) and signs (dryness, cracking, scaling, shedding/flaking, redness, and bleeding) of their psoriasis (0–10). Eligible patients (n=1,654) had comparable baseline PSSD scores. From Week 1, the anti-IL-17A cohort achieved significantly larger score improvements in PSSD summary scores and a higher proportion of patients showed clinically meaningful improvement (CMI) compared to the other biologics cohort through 12 weeks, the researchers report. Lower PSSD scores were associated with a greater proportion of patients reporting their psoriasis as no longer impacting their quality-of-life (DLQI 0,1) and a high level of clinical response (PASI 100). They note that results also indicate a relationship between an early CMI in PSSD score at Week 2 and PASI 100 score at Week 12.
Results showed that treatment with anti-IL-17A biologics, particularly ixekizumab, resulted in rapid and sustained patient-reported improvements in psoriasis symptoms and signs compared to other biologics in a real-world setting.
The Psoriasis Study of Health Outcomes (PSoHO) is an international, prospective, non-interventional study that compares the effectiveness of anti-interleukin (IL)-17A biologics versus other biologics, together with pairwise comparisons of ixekizumab versus five individual biologics in patients with PsO.
Comment: This is a very advanced international registry that compares IL-17 with other biologics to determine health outcomes. In addition to DLQI, PSSD score is an interesting measure that looks at psoriasis symptoms and signs according to the patient and not the physician. Although the DLQI can determine how a patient interacts with their social media or family environment, the Psoriasis Signs and Symptoms Diary allows patients to express the discomfort they may have directly in their skin and the signs they observe.
This way of measuring is different than what we are used to in terms of improvement of quality of life, but it looks at what the patient experiences on a day-to-day basis. It is certainly an important factor. In Australia, the guidelines determine that intractable itch is an indication for a biologic along with moderate to severe skin disease. This itch factor has not been instituted in many other countries, if any, and Australia should be commended for recognizing the importance of this symptom. This study showed that IL-17s—because of their rapid improvement compared to other biologics—could be a benefit for those who suffer from many symptoms of psoriasis.

Exposure to biologic therapy before and during pregnancy in patients with psoriasis
The authors conducted a systematic review and meta-analysis of the safety of biologics for psoriasis in women of child-bearing age. They aimed to describe the characteristics and pregnancy outcomes in women with psoriasis exposed to biologics within three months before or during pregnancy, and to estimate the pooled prevalence of spontaneous, elective, and total abortions, and congenital malformations in the newborns (J Eur Acad Dermatol Venereol June 1, 2023; doi: 10.1111/jdv.19238).
Literature searches were performed in the PubMed, Embase, Scopus and Web of Science databases up to Apr. 14, 2022 that reported pregnancy outcomes in women exposed to biologics indicated for psoriasis during the pre-gestational and/or gestational period. Studies focusing on rheumatologic or gastroenterological immune-mediated inflammatory diseases were excluded.
A total of 51 observational studies that involved 739 pregnancies exposed to approved biologics for psoriasis were assessed. Administration was mostly (70.4%) limited to the first trimester, and the most common therapy was ustekinumab (36.0%). They determined the estimated prevalence of miscarriage was 15.3% (95% confidence interval [CI] 12.7–18.0) and elective abortions was 10.8% (95% CI 7.7–14.3). Congenital malformations occurred in about 3.0% (95% CI 1.6–4.8) of live births exposed to biologics during pregnancy. According to the researchers, these rates are similar to the general population and suggest that biologic therapies for psoriasis used during pregnancy and/or conception not seem to be associated with an increased risk of miscarriage/abortion or congenital malformations.
Comment: This extremely important observational study revealed that biologics are generally safe in pregnancy. It is not surprising that ustekinumab was the most common therapy as it has been determined to be an extremely safe biologic compared to the TNF inhibitors, and has wide usage internationally. The important thing is that the rates of any pregnancy concerns are similar to the general population. However, pregnancy is an extremely sensitive health situation which can cause anxiety among parents and families as well as treating physicians when a patient is on an injectable medication such as a biologic for moderate to severe psoriasis. Certolizumab has been shown in clinical studies to be safe throughout all of pregnancy due to its unique structure. In females of childbearing potential, I recommend certolizumab for these patients. However, if a patient is already on a biologic and doing well, I do not suggest a switch to certolizumab during pregnancy. Generally, psoriasis is inactive during pregnancy and therefore quite often biologics may not be required throughout the 40 weeks. However, anxiety still persists and quite often it is recommended that biologics be discontinued in the third trimester if the patient is not on certolizumab. This study is reassuring to those that continue with other biologics throughout pregnancy.
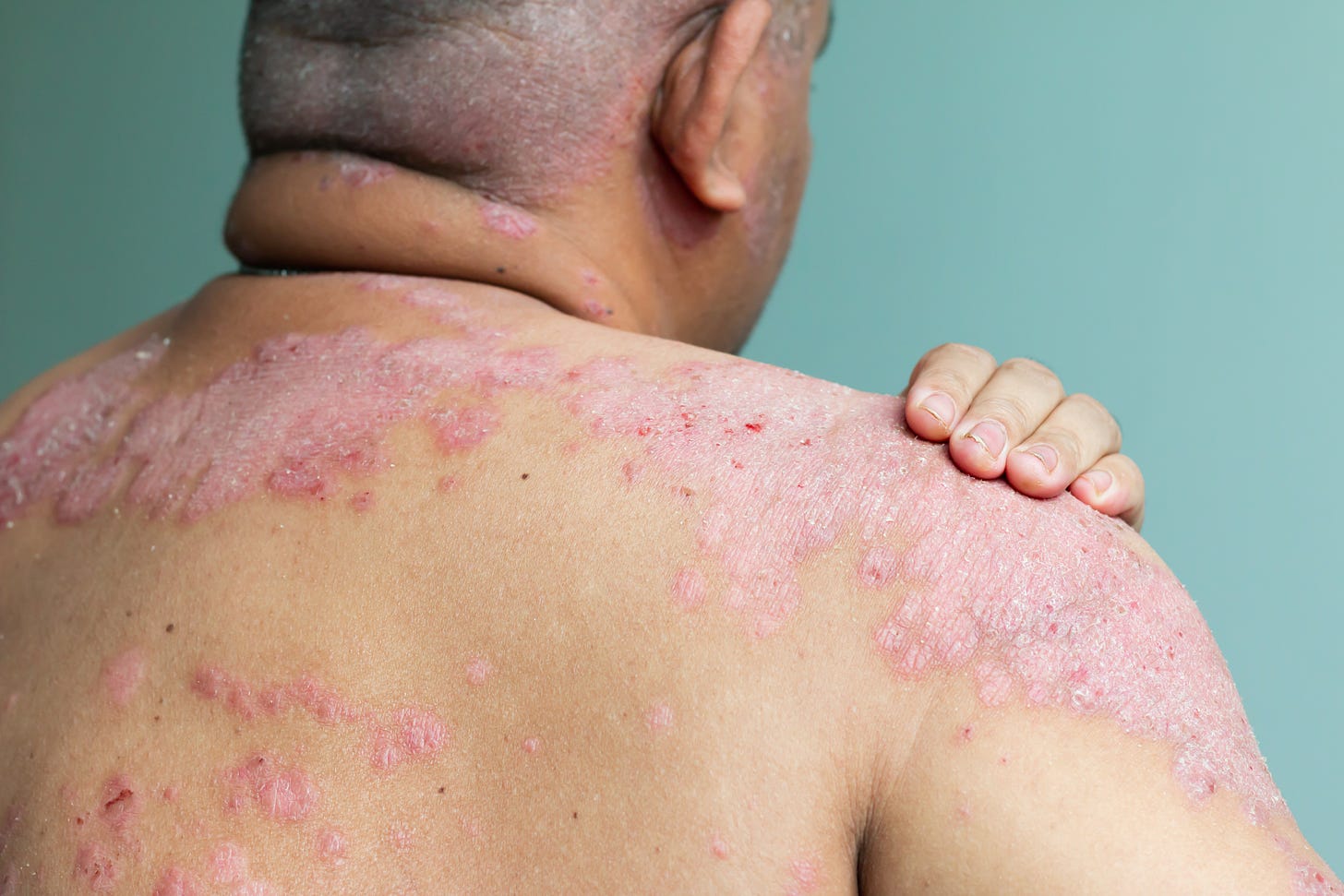
If you find the contents of this newsletter interesting, please check out the Vender on Psoriasis podcast. It’s available at Apple iTunes, Stitcher, Spotify, or wherever you get your podcasts.
Dr. Ron Vender is a Hamilton-based certified dermatologist with over 30 years of clinical practice experience and over 100 clinical trials in psoriasis. He is founder and director of Dermatrials Research Incorporated and Venderm Consulting, specializing in treatments and management solutions for individuals with psoriasis.
Subscribe to The Chronicle of Skin & Allergy
Established in 1995, The Chronicle of Skin & Allergy is a scientific newspaper providing news and information on practical therapeutics and clinical progress in dermatologic medicine.
To apply for a complimentary* subscription, please email health@chronicle.org with your contact information or click the link below.






